MedDRA® DATA RETRIEVAL AND
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Release 3.8 |
1 September 2014
Disclaimer and Copyright Notice This document is protected by copyright and may be used, reproduced, incorporated into other works, adapted, modified, translated or distributed under a public license provided that ICH's copyright in the document is acknowledged at all times. In case of any adaption, modification or translation of the document, reasonable steps must be taken to clearly label, demarcate or otherwise identify that changes were made to or based on the original document. Any impression that the adaption, modification or translation of the original document is endorsed or sponsored by the ICH must be avoided. The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document. The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder. MedDRA® trademark is owned by IFPMA on behalf of ICH |
The Medical Dictionary for Regulatory Activities terminology (MedDRA) was designed for sharing regulatory information for human medical products. However, unless users achieve consistency in how they assign terms to verbatim reports of symptoms, signs, diseases, etc., and in methods for data retrieval and evaluation, use of MedDRA cannot have the desired harmonising effect in the exchange of coded data.
MedDRA is a large terminology with very specific (“granular”) terms called Lowest Level Terms (LLTs) that serve to accurately record the reporter’s words (verbatim term). LLTs are generally synonyms linked to their parent terms known as Preferred Terms (PTs). PTs are also relatively specific and large in number.
While a highly granular terminology such as MedDRA reduces the need for interpretation at data entry, it impacts the processes of data retrieval, sorting and presentation necessary for support of drug development, pharmacovigilance and risk management. The hierarchical structure of MedDRA facilitates data retrieval by providing grouping terms (High Level Terms [HLTs] and High Level Group Terms [HLGTs]) that aggregate the very specific terms used for coding into broader medical categories. MedDRA’s multiaxiality (assignment of a PT to more than one System Organ Class [SOC]) allows flexibility in data retrieval via primary and secondary paths. Whilst grouping terms and multiaxiality permit a reasonable first approach to data retrieval, the complexity of MedDRA requires guidance to optimise the results.
This Data Retrieval and Presentation: Points to Consider (DRP:PTC) document is an ICH-endorsed guide for MedDRA users. It is updated in step with new MedDRA versions and is a companion document to MedDRA. It was developed and is maintained by a working group charged by the ICH Steering Committee. The working group consists of regulatory and industry representatives of the European Union, Japan, and the United States, as well as representatives from the Canadian and Korean regulatory authorities, the MedDRA Maintenance and Support Services Organization (MSSO) and the Japanese Maintenance Organization (JMO) (see Appendix, Section 6.2 for list of members).
The principles described in this document are most effective when used in conjunction with the principles described in the MedDRA Term Selection: Points to Consider document for data entry (coding). This document provides data retrieval and presentation options for either industry or regulatory purposes. Although MedDRA includes some data retrieval tools, this document addresses data retrieval in a broader context.
Examples in this document are based on MedDRA Version 17.1; they are intended to facilitate reader understanding and are not intended to imply regulatory requirements.
Figures referenced in the text are found in the Appendix, Section 6.3.
1.1 – Objectives of this Document
The objective of the DRP:PTC document is to demonstrate how data retrieval options impact the accuracy and consistency of data output. For example, certain drugs or therapeutic areas may need a customised approach for data output. Options for data input described in the MedDRA Term Selection: Points to Consider document – or in organisation-specific coding guidelines – should also be taken into consideration.
Organisations are encouraged to document their data retrieval and output strategies, methods and quality assurance procedures in organisation-specific guidelines which should be consistent with this DRP:PTC document.
1.2 – Reasons to Use MedDRA
MedDRA is used to report adverse reaction/adverse event (AR/AE) terms in individual case reports – both on paper or electronically. Its structure allows for aggregation of those reported terms in medically meaningful groupings to facilitate analysis of safety data. MedDRA can also be used to list AR/AE data in reports (tables, line listings, etc), compute frequencies of similar AR/AEs, and capture and analyse related data such as product indications, investigations, and medical and social history.
1.3 – How to Use this Document
The principles described in this document apply to all data encoded with MedDRA with a focus on aggregated data. This document does not address the use of MedDRA for single case reporting, labeling, medical evaluation and statistical methodology.
This Points to Consider document aims to help all MedDRA users, since the MedDRA terminology itself contains no specific guidelines for its use. The document provides a framework to foster consistent use of MedDRA for data analysis and presentation for medically meaningful review and analysis of
clinical data.
This document describes the features of MedDRA and highlights the impact of MedDRA’s structure, rules and conventions on data output. Examples and options described in the document are not intended to communicate specific regulatory reporting requirements or address specific database issues. This document cannot address every situation, therefore, medical judgment should always be applied.
The document is not a substitute for MedDRA training. It is essential for users to have knowledge of MedDRA’s structure and content. For optimal use of MedDRA, one should refer to the MedDRA Introductory Guide, the Introductory Guide for Standardised MedDRA Queries (SMQs) (see Appendix, Section 6.1,) and the MedDRA Term Selection: Points to Consider document.
Section 2 – GENERAL PRINCIPLES
2.1 – Quality of Source Data
High quality data output occurs when the quality of the information originally reported is maintained with consistent and appropriate term selection. Organisations should pursue continuous oversight of data quality. Data quality issues are also addressed in the MedDRA Term Selection: Points to Consider document.
2.1.1 Data conversion considerations
Give special consideration to the method used to convert data from other terminologies into MedDRA. The methods used can impact retrieval and presentation strategies.
Ø Method 1 – Data converted from legacy terminology terms to MedDRA
• Results will reflect the specificity of the previous terminology
• The benefits of the greater specificity of MedDRA are not attained
Example
| Reported | Legacy Term | MedDRA Term |
| Gastrointestinal ischaemia | Gastrointestinal Disorder | Gastrointestinal disorder |
Ø Method 2 – Data converted from the original reported terms (verbatim terms) to MedDRA terms
Example
| Reported | Legacy Term | MedDRA Term |
| Gastrointestinal ischaemia | Gastrointestinal Disorder | Gastrointestinal ischaemia |
Document the data conversion method used, including the date of the conversion.
2.1.2 Impact of data conversion method
Combining the two conversion methods described above can affect interpretation of data output.
Example
| Data Output with Combined Data Conversion Methods |
| If data have been converted directly from legacy terminology terms to MedDRA terms (Method 1), and if newly acquired data are coded directly from reported terms to MedDRA, the resulting differences in specificity could make interpretation difficult. |
When designing a search strategy, it may be useful to examine the reported terms for data converted using Method 1. If the search has been based on specific MedDRA terms, data previously coded to non-specific terms may be otherwise overlooked.
Example
| Impact of Method 1 Conversion on Search Strategy |
| If searching with MedDRA PT Gastrointestinal ischaemia, cases of gastrointestinal ischaemia coded with the legacy term Gastrointestinal disorder would be missed. In this case, it would be important to know the date of the legacy data conversion. |
To conduct a search requiring this level of detail, it might be necessary to review or recode from the reported terms. For legacy data, this information might be found in fields other than those for ARs/AEs.
2.2 – Documentation of Data Retrieval and Presentation Practices
It is important to document MedDRA term selection conventions, data retrieval and output strategies (including SMQs and other queries) and quality assurance procedures. Organisation-specific strategies should be consistent with the Points to Consider documents and should include:
• MedDRA version used for the search
• Search strategy methods (sufficiently detailed to be reproducible)
• Version update processes
• Processes for creating and maintaining customized MedDRA queries
2.3 – Do Not Alter MedDRA
MedDRA is a standardised terminology with a pre-defined term hierarchy that should not be altered. Users must not make ad hoc structural alterations to MedDRA, including changing the primary SOC allocation; doing so would compromise the integrity of this standard. If terms are found to be incorrectly placed in the MedDRA hierarchy, a change request should be submitted to the MSSO.
2.4 – Organisation-Specific Data Characteristics
Although MedDRA is a standardised terminology, different organisations have implemented it is various ways. It is important to understand organisation-specific data characteristics and implementation strategies.
Each organisation should have access to a MedDRA specialist to provide expert advice and who has the knowledge of the following database characteristics:
• Database structure (how the MedDRA hierarchy is stored and used)
• Data storage (e.g., level of term, synonym/reported term)
• Data conversion from other terminologies (if applicable)
• Coding practices over time
Example
| Impact of Coding Practices Over Time |
| Consider the impact of gender-specific terms when comparing MedDRA coded data to data coded with an older terminology that may not have had corresponding gender-specific terms. If the prior terminology had only a single, gender-neutral term for “breast cancer”, consider the impact of selecting gender-specific breast cancer terms in MedDRA for current data. |
• Limitations or restrictions
Example
| Output or Display of Multiaxial PTs |
| Do not assume that PTs in their secondary SOC locations will be seen when searching in a specific HLT or HLGT since the database configuration may not allow output or display by the secondary path. |
• Term selection principles used
o Selecting more than one term when coding a medical condition increases counts of terms.
o Selecting a diagnosis term only (and not terms for signs and symptoms) reduces the counts of terms.
o The adverse event profile resulting when both diagnosis and signs/symptoms terms are coded may appear different than when the diagnosis only is coded. Always consider the organisation’s coding conventions when using or comparing data from other databases (e.g., co-developing or co-marketing partners, regulatory authorities).
2.5 – Characteristics of MedDRA that Impact Data Retrieval and Analysis
MedDRA’s structure, rules and conventions are detailed in the MedDRA Introductory Guide.
Keep the following MedDRA characteristics in mind for data retrieval and presentation:
2.5.1 Grouping terms (HLTs and HLGTs)
The HLT and HLGT levels are an additional tool for data analysis and retrieval as they provide clinically relevant groupings of terms.
Example
| Cardiac Arrhythmias |
HLGT Cardiac arrhythmias
HLT Cardiac conduction disorders
HLT Rate and rhythm disorders NEC
HLT Supraventricular arrhythmias
HLT Ventricular arrhythmias and cardiac arrest |
2.5.1.1 Review terms within a grouping term
Review terms within the HLGT or HLT of interest to be sure that all terms therein are suited for the purpose of the output.
Example
| Blood Pressure Terms |
HLT Vascular tests NEC (incl blood pressure)
PT Blood pressure
PT Blood pressure abnormal
PT Blood pressure decreased
PT Blood pressure increased
Note that terms for increased and decreased blood pressure are grouped under a single HLT which also includes PTs for pulmonary arterial pressure, vascular resistance, haemodynamic tests, etc.
|
2.5.2 Granularity
MedDRA PTs are more specific (“granular”) than comparable terms in other terminologies. Figure 1 illustrates how data coded to a single concept from another terminology may be coded to several PTs in MedDRA.
Related events that may have been represented by a single term in another terminology may be represented by more than one MedDRA PTs. The potential impact of this on signal detection should be kept in mind.
2.5.3 Multiaxiality
Multiaxiality means that a PT may exist in more than one SOC. This allows terms to be grouped in different, but medically appropriate, ways (e.g., by etiology or organ system). Each PT is assigned one primary SOC; all other SOC assignments for that PT are called “secondary”. Having a single primary SOC prevents double counting of events when outputting data from all SOCs.
All possible secondary SOC assignments for any given PT may not be present in MedDRA. However, new or revised SOC assignments can be created as a result of the change request process.
2.5.3.1 Primary SOC assignment rules
Primary SOC assignment rules are described in the MedDRA Introductory Guide. These rules affect the way terms are placed in MedDRA and determine their data display by SOC. Because these rules allow for terms related to a particular medical condition to be in more than one SOC, users should be familiar with the general structure and content of all MedDRA SOCs to be sure that data are not overlooked.
Example
| Type of Disorder | Primary SOC Rule | Example | Comment |
| Congenital | All terms for congenital disorders have as their primary SOC assignment SOCCongenital, familial and genetic disorders | PT Congenital absence of bile ducts has a primary SOC assignment of SOC Congenital, familial and genetic disorders and a secondary SOC assignment of SOC Hepatobiliary disorders | The secondary SOC assignment for these terms is their “site of manifestation” SOC |
| Neoplastic | All terms for malignant and benign neoplasms (except cysts and polyps) have as their primary SOC assignment SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps) | PT Skin cancer has a primary SOC assignment of SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps) and a secondary SOC assignment of SOC Skin and subcutaneous tissue disorders | Cyst and polyp terms are an exception to this rule. The primary SOC assignment for cyst and polyp terms is the “site of manifestation” SOC, and the secondary SOC is SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
| Infectious | All terms for infectious disorders have as their primary SOC assignment SOC Infections and infestations | PT Enterocolitis infectious has a primary SOC assignment of SOC Infections and infestations and a secondary SOC assignment of SOC Gastrointestinal disorders | The secondary SOC assignment for these terms is their “site of manifestation” SOC |
If a PT links to more than one of these three SOCs, the following priority is used to determine the primary SOC:
• SOC Congenital, familial and genetic disorders
• SOC Neoplasms benign, malignant and unspecified (incl cysts and polyps)
• SOC Infections and infestations
2.5.3.2 Non multiaxial SOCs
Terms in the following three SOCs do not have multiaxial links:
SOC Investigations
SOC Surgical and medical procedures
SOC Social circumstances
This is important when designing queries and other retrieval strategies because one cannot rely on multiaxiality to locate all terms of interest in MedDRA.
Example
| Impact of Non Multiaxial SOCs on Data Queries |
| When querying a database for events or cases of thrombocytopenia, data coded to PTs in SOC Blood and lymphatic system disorders is a logical starting point. Additionally, data coded to terms in SOC Investigations – such as PT Platelet count decreased – and data coded to terms in SOC Surgical and medical procedures - such as PT Platelet transfusion – could also be of interest. Neither of these PTs has a link to SOC Blood and lymphatic system disorders. Failure to consider data coded in the non multiaxial SOCs could lead to incomplete analysis of thrombocytopenia. |
As noted above, terms for test results are in SOC Investigations and do not have multiaxial links to terms for corresponding medical conditions. Keep this in mind when reviewing tables and data listings of MedDRA coded data.
Example
| Terms for Test Results in SOC Investigations |
| When querying a database for events or cases of hepatic abnormalities, data coded to PTs in SOC Hepatobiliary disorders is a logical starting point. Additionally, data coded to terms in SOC Investigations – such as PT Liver function test abnormal – and data coded to terms in SOC Surgical and medical procedures - such as PT Liver transplant – could also be of interest. Neither of these PTs has a link to SOC Hepatobiliary disorders. Failure to consider data coded in the non multiaxial SOCs could lead to incomplete analysis of hepatic abnormalities. |
Figure 2 further illustrates the impact of data coded as test results vs. the corresponding medical condition.
2.5.3.3 Clinically related PTs
Clinically related PTs might be overlooked or not recognized as belonging together because they might be in different groupings within a single SOC or they may be located in more than one SOC (see Section 2.5.3).
Example
| Similar Skin Conditions in Different Groupings |
HLGT Epidermal and dermal conditions
HLT Bullous conditions
PT Stevens-Johnson syndrome
PT Toxic epidermal necrolysis
HLT Exfoliative conditions
PT Dermatitis exfoliative
PT Dermatitis exfoliative generalised
PT Nikolsky's sign
PT Skin exfoliation |
The frequency of a medical concept may be underestimated if the above points are not considered; this may impact interpretation of data (see Section 3.2).
MedDRA SOCs group terms by body systems, aetiologies and specialised purposes. Data may be coded to terms in SOCs that had not been anticipated by the user. Keep in mind the potential impact of multiaxiality on frequencies of the medical condition of interest.
Example
| Preferred Term | Primary SOC |
| Post procedural haemorrhage | Injury, poisoning and procedural complications |
| Chest pain | General disorders and administration site conditions |
2.6 – MedDRA Versioning
MedDRA is updated twice yearly. Version “X.0” contains both simple and complex changes; version “X.1” contains only simple changes.
Organisations should be aware of the types of MedDRA changes for their possible impact on data output.
| Types of MedDRA Changes | |
| Simple Changes | Complex Changes |
| Add a PT (new medical concept) Move an existing PT from one HLT to another Demote a PT to LLT level Add or remove a link to an existing PT Add an LLT Move an existing LLT from one PT to another Promote an LLT to PT level Make a current LLT non-current or a non-current LLT current Changing the primary SOC allocation Changes to SMQs |
Add or change multiaxial links Add new grouping terms Merge existing grouping terms Restructure a SOC |
Both simple and complex changes impact retrieval and presentation strategies. Users should read the documentation provided with each MedDRA release, especially the What’s New document. The MSSO and JMO provide tools to assist the user in comparing the changes between MedDRA versions. The Version Report (provided by the MSSO and JMO) is a spreadsheet listing all changes between the current version of MedDRA and the one previous to it; this spreadsheet is provided with each new release of MedDRA. The MSSO also provides the MedDRA Version Analysis Tool (MVAT) that facilitates identification and understanding of the impact of changes between any two MedDRA versions, including non-consecutive ones (see Appendix, Section 6.1 of this document; also, see Section 4.1.1 of the MedDRA Term Selection: Points to Consider document).
Organisations should plan and document their strategy for handling MedDRA version updates. When planning or performing data retrieval and presentation, the MedDRA version used should be documented.
Keep in mind that MedDRA changes may impact previous data retrieval approaches and results, including event frequencies.
Example
| Impact of Version Changes – Demoted PT |
| PT Chest tube insertion was included in a query developed using terms in MedDRA Version 17.0. If the query had been re-run on data using MedDRA Version 17.1, these events would not have been found at the PT level because PT Chest tube insertion had been demoted to an LLT and linked to PT Thoracostomy. See Figure 3. |
Example
| Impact of Version Changes – Change of Primary SOC Assignment |
| PT Urinary bladder rupture had a primary link to SOC Injury, poisoning and procedural complications and a secondary link to SOC Renal and urinary disorders in MedDRA Version 17.0. In Version 17.1, the primary SOC assignment was changed to SOC Renal and urinary disorders and the secondary assignment to SOC Injury, poisoning and procedural complications. In a primary SOC output of data, PT Urinary bladder rupture will seem to have “disappeared” from SOC Injury, poisoning and procedural complications. |
Terms used to construct queries should be in the same MedDRA version as the data being queried. An organisation’s legacy data may be coded in more than one version of MedDRA. New terms may have been included in a new query built in MedDRA Version 17.1; depending upon the organisation’s versioning method, these new terms might not be present in the older data. This could lead to search results that are incomplete.
A search built with terms of an earlier MedDRA version (e.g., used previously on a now closed study) might not identify all relevant data in an integrated safety summary (ISS) containing data coded in a later version of MedDRA. Queries stored in an organisation’s system should be updated to the appropriate version of MedDRA before using them on new data.
Advice on how an organisation should handle new MedDRA versions is not within the scope of this document (see MedDRA Term Selection: Points to Consider, Appendix 4.1). Some databases may contain data of multiple studies coded in different versions of MedDRA. This may impact aggregation of those data (e.g., in an ISS). Refer to MSSO documents on versioning options for clinical trial and post-marketing data for more information (see Appendix, Section 6.1).
Section 3 – GENERAL QUERIES AND RETRIEVAL
3.1 – General Principles
Data retrieval is performed for summary and analysis of clinical trial data, pharmacovigilance, medical information questions and for a number of other purposes. The search strategies, methods and tools used to retrieve data might differ based on the intended use of the output.
A general approach for data retrieval is outlined in the chart below.
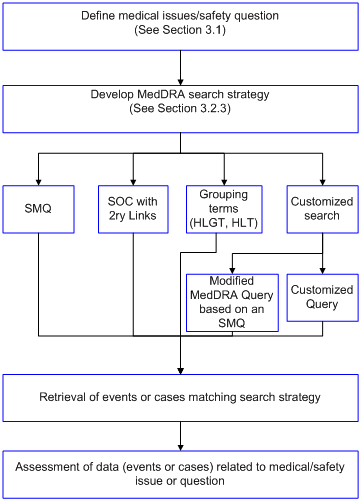
Prior to data retrieval, there may be known or potential safety issues that need detailed investigation. Information from pre-clinical studies, clinical trials post-marketing surveillance, class effects of similar products, and regulatory queries may identify areas of possible focus; these may affect the strategy for aggregating search terms, the methodology, and the way data are displayed.
Be aware of database characteristics, organisation-specific data entry conventions, data sources, the size of the database, and the version of MedDRA used for coding all data. Archived searches may be available to the user, especially those used in pharmacovigilance; these may be suitable for
use if updated.
When presenting adverse event data, it is important to display and to group related events (i.e., events that represent the same condition of interest) so that the true occurrence rate of an event is not obscured. Search strategies should be documented. The search output alone may not suffice for data assessment (e.g., frequency of a condition). Search results should be evaluated against the question originally posed.
Sorting related events into categories can be challenging. A search that is too narrowly focused might exclude events of potential relevance; a search that is too broad might make it difficult to identify a trend or signal. Careful interpretation is required when grouping terms that correspond to a potential event or medical condition for analysis (whether a syndrome or not). The purpose is to identify trends that may require further analysis, including review of individual cases. For complex queries, create a data analysis plan including a definition of the medical condition of interest. An interdisciplinary discussion might be helpful to identify the most suitable methods and tools relevant
to the query.
These principles may apply to the types of searches listed in the table below:
Example
| Types of Searches – Application of General Principles |
| Safety profile overview in a summary report, Periodic Safety Update Report (PSUR), ISS, etc. Comparing frequencies of ARs/AEs reporting rates for spontaneous reports or incidence for studies) Analysis of a specific safety concern Identifying patient subpopulations at risk (search of medical history) |
3.1.1 Grapical displays
Graphical displays can be useful especially with large datasets. Such displays allow quick visual representation of potential signals. Organisations are encouraged to use graphs for data display. Histograms, bar charts, and pie charts can be useful as can more complex, statistically-derived displays (e.g., data mining algorithms). Examples of these types of displays are in the Appendix, Section 6.3.
3.1.2 Patient subpopulations
Paediatric and gender-specific adverse event terms lists – available on the MedDRA and JMO websites – may aid in data retrieval for these subpopulations (see Appendix, Section 6.1). However, it is necessary to refer to individual database fields for demographics.
3.2 – Overall Presentation of Safety Profiles
The aims of an overall safety profile presentation are to:
• Highlight distribution of ARs/AEs
• Identify areas for in depth analysis
Present the data in a way that allows for easy recognition of patterns of terms potentially related to the relevant medical conditions. There are various ways to do this ranging from a full listing of terms to sophisticated statistical approaches such as data mining techniques (for reference, see ICH E2E: Pharmacovigilance Planning Document; listed in the Appendix, Section 6.1).
Historically, the standard approach has been to display data by Body System (or System Organ Class) and Preferred Term corresponding to SOCs and PTs in MedDRA. Due to MedDRA’s unique characteristics (multiaxiality, granularity), this PT-SOC approach may need to be augmented with other types of data outputs (e.g., secondary SOC output, display by grouping terms [HLTs, HLGTs], etc.), depending on the reason for the output. For example, if a number of reports describe a similar medical condition, they could be represented by:
• Many different PTs (dilution of signal)
• Different grouping terms
• Different SOCs
SOCs where the user would not intuitively expect them (e.g., SOC General disorders and administration site conditions, SOC Pregnancy, puerperium and perinatal conditions, SOC Injury, poisoning and procedural complications, SOC Infections and infestations). See examples in the table below:
Example
| PTs with Primary SOC General disorders and administration site conditions and Secondary SOC Cardiac disorders |
PT Chest discomfort
PT Chest pain
PT Oedema peripheral
PT Sudden death
PT Localised oedema
PT Oedema due to cardiac disease
PT Peripheral oedema neonatal
PT Cardiac death |
3.2.1 Overview by primary System Organ Class
This overview is recommended as a first step in data retrieval and for planning of further analysis.
Display of all data ensures that all events will be seen and may be useful to identify data clusters by SOC. If the hierarchy is also displayed, clusters may occur at the HLGT or HLT levels. For a small dataset, this display by primary SOC may be all that is necessary.
Ø Objectives:
• Include all events (none are omitted)
• Display all data in the entire MedDRA hierarchy
Ø Method:
The primary SOC view including HLGTs, HLTs and PTs can be used for standard tables (clinical trials and post-marketing data) and for cumulative summaries (post-marketing data). Line listings (both clinical and post-marketing data) can also be displayed by primary SOC and PT. Depending on the reason for the output, it might be beneficial to use the primary SOC and PT display; for large datasets, display by SOC and by grouping terms (HLGTs and HLTs) may be preferable. Figure 4 is an example of such an output.
The Internationally Agreed Order of SOCs was developed for consistency irrespective of language or alphabet (see Figure 5). The SOC order was based upon the relative importance of each SOC in AR/AE reports. Use of the Internationally Agreed Order may be applicable to certain regulatory functions, e.g., the SPC guideline (see the MedDRA Introductory Guide and MedDRA ASCII files). Organisations that share data should agree on the order of SOCs when preparing data for presentation.
Data displays in tables or in graphical presentations may facilitate understanding by the viewer. Figures 6, 7 and 8 are examples of such displays.
Figures 9a and 9b display data for one compound in two patient populations. Within each patient population, the reports are split by SOC and by reporter. The upper bar of each pair represents numbers of reports from consumers (blue), and the lower bar represents reports from health care professionals (red). If further detail is needed, adverse events can be displayed by PT with decreasing frequency.
In depth analysis requires medical expertise to define terms that should be aggregated.
Ø Benefits:
• Provides an overview of data distribution; helps identify areas of special interest that may need in depth analysis
• Grouping terms aggregate related PTs, facilitating identification of medical conditions of interest
• A PT will be displayed only once, preventing over-counting of terms
• A primary SOC overview may be the only form of data display necessary for a small dataset
Ø Limitations:
• Because it is based on a PT-to-primary SOC assignment, there may be incomplete groupings of terms for a medical condition or syndrome as such terms may be distributed among different SOCs
• Events may not be found where the user expects them due to MedDRA placement rules
• Potential for a lengthy data output when applied to large datasets
3.2.2 Overall presentations of small datasets
When the safety profile consists of a small list of PTs (e.g., early in clinical development), a display of these PTs may be adequate. Figure 10 is an example of this.
3.2.3 Focused searches
Focused searches may be useful for further investigation of medical concepts of interest. For example, a focused search may be used to determine the number of cases or events of interest in response to a regulatory query.
In certain situations, such as those listed below (note that this list is not all-inclusive), users may wish to design a specific search in addition to the Overview by Primary System Organ Class (see Section 3.2.1).
• Further examination of clusters seen in Primary SOC output
• Previously identified safety concerns (e.g., known class effects, results from toxicology and animal studies, etc.)
• Monitoring events of special interest
• Responding to regulatory queries
Below are listed options for focused search approaches. The order of applying these approaches may depend on resources, expertise, systems or other factors.
3.2.3.1 Focused searches by secondary SOC assignments
This focused search augments the Overview by Primary System Organ Class (see Section 3.2.1) by addressing secondary SOC assignments, thus providing a more comprehensive view of the data and taking advantage of MedDRA’s multiaxiality.
Ø Method:
The method used for a focused search by secondary SOC assignment may depend on the database characteristics of the organisation.
Options include:
• Query of the SOC, HLGT and HLT levels to include both the primary and secondary SOC assignments in the display
• Output PTs in their secondary SOC locations programmatically (see Figure 11)
• If the database does not allow automated output by secondary SOC, then perform the query using available processes (e.g., programming a list of all individual PTs in the primary and secondary SOC locations)
Example
| Programming a List of PTs in Primary and Secondary SOC Locations |
SOC Eye disorders
HLGT Vision disorders
HLT Visual pathway disorders
PT Chiasma syndrome
PT Optic nerve disorder (primary SOC location)
PT Optic neuropathy (primary SOC location)
PT Toxic optic neuropathy (primary SOC location)
PT Visual cortex atrophy
PT Visual pathway disorder
3 of 6 PTs are primary to SOC Nervous system disorders |
Ø Benefits:
Multiaxial links enhance the utility of the grouping terms. This method overcomes the primary SOC limitations as described under Section 3.2.1.
Ø Limitations:
• Still displays only terms that are represented in one SOC or HLGT/HLT which may not include all terms related to a medical condition
• This method of display of PTs by both primary and secondary SOC assignments could lead to double counting of cases/events)
Section 4 – STANDARDISED MedDRA QUERIES
4.1 – Introduction
Standardised MedDRA Queries (SMQs) were created to standardise identification and retrieval of safety data.
SMQs are a joint effort of the Council for International Organizations of Medical Sciences (CIOMS) and ICH (including MSSO and JMO) representing both industry and regulatory authorities. An SMQ is a grouping of terms from one or more SOCs that relate to a defined medical condition or area of interest. The terms included relate to signs, symptoms, diagnoses, syndromes, physical findings, laboratory and other physiologic test data, etc. that are associated with the medical condition or area of interest.
Users should carefully read the Introductory Guide for Standardised MedDRA Queries (SMQs) before applying an SMQ to fully understand the scope of the SMQ and to properly apply search options such as algorithms and weightings.
4.2 – SMQ Benefits
As with all MedDRA-based queries, users of SMQs should be aware of several factors that may influence data retrieval including database characteristics, data conversion processes, coding conventions, and MedDRA versioning. For more details, see Section 3.1.
Ø SMQ benefits include:
• Application across multiple therapeutic areas
• Validated reusable search logic
• Standardised communication of safety information
• Consistent data retrieval
• Maintenance by MSSO and JMO
• SMQs do not cover all medical topics or safety issues
• SMQs evolve and undergo further refinement even though they have been tested during development
4.4 – SMQ Modifications and Organisation-Constructed Queries
If any modifications are made to term content or structure of an SMQ, it can no longer be called an “SMQ” but it should instead be referred to as a “modified MedDRA query based on an SMQ”. See Section 5.1 for further details on SMQ modification.
Under no circumstances should a query constructed for the specific need of an organisation be called an “SMQ” by its originator. This is to ensure that there is no confusion with the ICH-endorsed SMQs applied by other MedDRA users. Any alternate name for the organisation-constructed query is acceptable as long as it could not be potentially confused with an ICH-endorsed SMQ.
4.5 – SMQs and MedDRA Version Changes
Each SMQ relates to a specific MedDRA version. SMQs are part of each new MedDRA release, are maintained by MSSO and JMO, and correspond to the terms present in that version of MedDRA. The SMQ version should always correspond to the MedDRA version of the data being searched.
As with all searches of MedDRA-based data, it is important to document the MedDRA and SMQ versions used.
Changes to SMQs that can occur with each MedDRA version include (but are not limited to) the following:
• Addition of PTs
• Inactivation of a PT (i.e., effectively “removing” a PT from an SMQ)
• Change of term scope (e.g., a narrow term becomes a broad term)
• Restructuring of an SMQ (e.g., change in the hierarchical position of an SMQ)
• Creation of a new SMQ
For a full description of the types of changes that can occur to SMQs, please refer to the MedDRA “Change Request Information” document (see Appendix, Section 6.1). Changes introduced with each new version are documented in the “What’s New” document for each MedDRA version. (The cumulative changes are contained within the ASCII files in the fields called “Term_addition_version” and “Term_last_modified_version”).
The MedDRA version of the SMQ and the coded data being searched should be the same because mismatches could produce unexpected results. For example, if an SMQ from an older version of MedDRA is applied to data coded in a more recent version, data coded to terms that are not present in the older SMQ would not be retrieved.
Example
| Consequence of Version Mismatch of Coded Data and SMQ |
| PTThyrotoxic cardiomyopathy was added to SMQ Cardiomyopathy in MedDRA Version 17.1. Using Version 17.0 of this SMQ – which does not contain this PT – would fail to identify cases coded to this term in a database using MedDRA Version 17.1. |
4.6 – SMQs – Impact of MedDRA Legacy Data Conversion
The conversion method for data originally coded in another terminology (e.g., COSTART) also impacts the application and output of SMQs. See Section 2.1.2, Impact of data conversion method.
4.7 – SMQ Change Requests
Users are encouraged to submit Change Requests to MSSO and JMO to improve the utility of SMQs. A justification (and possibly testing data) for a submitted Change Request must be provided. The MSSO may require more time to evaluate SMQ Change requests than regular MedDRA Change Requests. Before submitting an SMQ Change Request, users should review the SMQ documentation for inclusion and exclusion criteria of the SMQ.
4.8 – SMQ Technical Tools
The MSSO browsers (both the desktop and Web-based browsers) allow for searching and viewing the contents of SMQs and they include additional details such as the SMQ description (definition) and development notes. An Excel spreadsheet containing the terms in each production SMQ is available from MSSO and JMO (see Appendix, Section 6.1). This spreadsheet allows a user to transfer SMQ terms to query tools. File specifications related to SMQs are found in the “MedDRA Distribution File Format Document” supplied with each MedDRA version.
The MedDRA website has a list of some system tools that provide technical support for SMQs (see Appendix, Section 6.1).
4.9 – SMQ Applications
SMQs were developed to address the high granularity and unique features of MedDRA and to maximise the likelihood that all terms related to a specific medical condition of interest are identified.
The user should first review the list of available SMQs to determine which of them may be applicable to the question being asked. If an SMQ seems applicable, the user should check the documentation in the SMQ Introductory Guide to understand the purpose and definition of the SMQ. The user may also wish to review the term contents of the SMQ.
Following application of the selected SMQ on coded data, search results (i.e., retrieved data) should then be evaluated against the question originally posed. The search output alone may not be sufficient for data assessment (e.g., frequency of a condition). Define and document criteria for case evaluation.
Generally, more cases/events will be retrieved than will eventually be subjected to analysis due to “noise”. This is a more significant consideration for “broad” searches but in principle also applies to “narrow” searches (see Section 4.10.1).
4.9.1 Clinical trials
SMQs may be applied in the clinical trial setting – especially for aggregate data – where the safety profile has yet to be fully established. In this instance, most (if not all) available SMQs may be used, possibly on a routine basis.
Alternatively, a user can apply an SMQ (or SMQs) that relates to a previously identified area of interest (e.g., from pre-clinical data or class effect) for further evaluation.
Example
| Targeted Safety Study |
| When developing a data analysis plan for a targeted safety study, consider using the narrow terms of an SMQ to aggregate events of interest. |
4.9.2.1 Focused searches
A specific SMQ or a selection of SMQs may be used to retrieve relevant cases for subsequent medical review
Example
| Emerging Safety Signal |
| A company suspects an emerging signal of pancreatitis for a new HIV product. SMQ Acute pancreatitis can be applied to the data. |
4.9.2.2 Signal detection
The entire set of SMQs may be used on the database for signal detection. The user may wish to use the narrow terms or more specific levels of hierarchical SMQs (i.e., a sub-search SMQ) to minimise dilution of the signal.
4.9.2.3 Signal case alert
SMQs may be used to create a “watch list” (e.g., an automated notification system) to alert the user of incoming cases needing urgent review.
Example
| Single Case Alert |
| A medical issue of interest needs to be communicated to a regulatory authority as part of an agreed risk management plan. The SMQ narrow search or more specific levels of a hierarchical SMQ may be applied to identify potential cases of interest. |
4.9.2.4 Periodic reporting
SMQs may help aggregate relevant cases for ongoing review of specific safety issues in periodic safety reports. SMQs may also be used for other routine reviews of aggregate data (e.g., reports of lack of efficacy) in the context of a periodic report.
4.10 – SMQ Search Options
Some SMQs have options that may be used to refine a particular search. The most common option is use of narrow and broad search terms. By definition, a broad search includes both narrow and broad terms.
Some SMQs are hierarchical (i.e., contain one or more sub-searches). Other SMQs use algorithms, and in one case (SMQ Systemic lupus erythematosus), weightings are assigned to particular terms for signs, symptoms and laboratory results to help identify cases.
4.10.1 Narrow and broad searches
Most SMQs have narrow and broad PTs. The narrow PTs have a greater likelihood of identifying only events of interest (high specificity) while the broad terms are intended to identify additional possible events (high sensitivity). Some events retrieved by the broad search terms may, upon further review, not relate to the condition of interest. The user can select the scope of the search (narrow or broad) that is most applicable to the question being asked. Figure 12 is an example of output of narrow and broad searches.
When a compound is in early phase development or has only recently been marketed, it may be advisable to use the broad search.
Example
| Use of Broad Search |
| If evaluating an emerging signal of lactic acidosis using SMQ Lactic acidosis, narrow terms may be applied to identify events where the specific diagnosis has been reported; however, events of reported signs and symptoms would not be retrieved. If there is additional need to find cases where no specific diagnosis (but mainly signs and symptoms) have been reported, then a broad search (i.e., narrow + broad search terms) should be applied. |
4.10.2 Hierarchical SMQs
Several SMQs have a hierarchical structure (one or more levels of sub-searches of increasing specificity). The user can select the search that is most applicable to the question being asked or a combination of sub-search SMQs as needed.
The SMQ Introductory Guide has explanatory notes on the appropriate use of each hierarchical SMQ. An example of a hierarchical SMQ is illustrated below (SMQ Haematopoietic cytopenias).
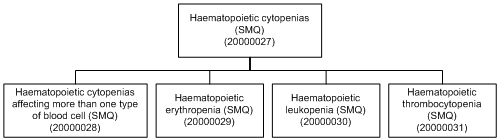
Example
| Use of SMQ Hierarchy |
| The medical condition of interest is thrombocytopenia. SMQ Haematopoietic cytopenias may be too inclusive because sub-searches for decreases of other hematopoietic cell lines (e.g., SMQ Haematopoietic leukopenia) are included. A user may wish to select only the sub-search SMQ Haematopoietic thrombocytopenia in this instance. |
4.10.3 Algorithmic SMQs
An algorithm provides for a combination of terms which – if retrieved in a single case – are more likely to identify a case of interest than isolated broad search terms (see table below). The broad terms of algorithmic SMQs are subdivided into categories that could be groupings of organ-specific signs or symptoms, laboratory terms, etc. (Note: the broad search categories are labeled B, C, D, etc.) Using an algorithm may reduce the amount of “noise” (i.e., non-relevant cases).
Using an algorithmic SMQ without applying the algorithm (i.e., simply applying the narrow and broad searches) will yield different results from those obtained using the algorithm.
Example
| Algorithmic SMQ (SMQ Anaphylactic reaction)* | ||
| Category B – Upper airway/Respiratory | Category C – Angioedema/Urticaria, etc. | Category D – Cardiovascular/Hypotension |
| Acute respiratory failure |
Allergic oedema | Blood pressure decreased |
| Asthma | Angioedema | Blood pressure diastolic decreased |
| Bronchial oedema | Erythema | Blood pressure systolic decreased |
Algorithm:
•Case = A (Narrow terms – not included in the table) •Or term from Category B and term from Category C •Or term from either Category B or Category C plus term from Category D |
||
* Not all terms in these categories are listed in the tabe
SMQ Systemic lupus erythematosus is an algorithmic SMQ with assigned weights for its included PTs (e.g., PT Pleural effusion = 3); a total weighted score greater than 6 suggests a case of interest.
Users should not assume that all software tools support algorithmic SMQs.
4.11 – SMQ and MedDRA Grouping Terms
Data retrieved using MedDRA Grouping terms (HLGTs, HLTs) may differ from those retrieved using a related SMQ.
Example
| Comparison – SMQ and Grouping Terms |
| Cardiac arrhythmia is a suspected issue(e.g., by review of a primary SOC output of all data). If events retrieved by using HLGT Cardiac arrhythmias are compared to those retrieved by SMQ Cardiac arrhythmias, more events may be retrieved by the SMQ because it includes additional terms from other SOCs such as SOC Investigations. |
Section 5 – CUSTOMISED SEARCHES
MedDRA allows for a variety of searching options as described above. However, there will be situations when a customised search is needed.
5.1 – Modified MedDRA Query Based on an SMQ
Do not modify the term content or structure of an SMQ unless there is a compelling reason to do so since altering it in any way makes it non-standard (see Section 4.4).
If an SMQ is modified in any way, it should be referred to as a “modified MedDRA query based on an SMQ”. All modifications to the original SMQ should be documented.
If a modified MedDRA query based on an SMQ is to be used on an ongoing basis, version updates and maintenance of the query are the responsibility of the organisation that created it.
Example
| Modified MedDRA Queries based on SMQs | |
| Additional PTs are needed | A product is being investigated for a possible safety signal of dementia, and the user wishes to use SMQ Dementia. For this particular product, PT Disturbance in attention may be needed. |
| Exclusion of PTs | An antipsychotic product is being investigated for potential QT prolongation and also has a well-described association with hypotension and fainting. When using SMQ Torsade de pointes/QT prolongation (broad search), the user may wish to exclude PT Syncope to prevent excess “noise” in data retrieval. |
| Changing the scope (narrow or broad) of an SMQ term |
A product is being investigated for the potential for hyperglycaemia and diabetes mellitus. SMQ Hyperglycaemia/new onset diabetes mellitus has PT Increased insulin requirement as a broad search term. For this query, it may be useful to include PT Increased insulin requirement in the narrow search |
5.2 – Customised Queries
Consider these points when constructing a customised query for MedDRA-coded data:
• Those responsiblle for constructing a customised query should
o Have medical knowledge
o Know the structure and characteristics of MedDRA (e.g., hierarchy, multiaxiality) and the general content of MedDRA groupings (SOCs, HLGTs, and HLTs)
o Understand the characteristics and structure of the data
• The specificy of the search should be defined.
• Initial focus should be on SOCs related to the condition of interest. For example, a customised search for a renal condition should start with SOC Renal and urinary disorders.
• The non multiaxial SOCs (SOC Investigations, SOC Surgical and medical procedures and SOC Social circumstances) should always be reviewed. Also, it may be useful to review terms in other SOCs that are not organ systems (e.g., SOC General disorders and administration site conditions, SOC Injury, poisoning and procedural complications and SOC Pregnancy, puerperium and perinatal conditions).
• It may be useful to identify relevant query terms by the following approaches:
o A “bottom-up” survey of MedDRA (terms at the LLT and PT levels initially)
o A “top-down” survey of MedDRA (starting at the SOC level and drilling down through the hierarchy)
• Consider looking at secondary links for multiaxial terms since additional relevant query terms could be found. For example, PT Dyspnoea can be found with other respiratory symptoms PTs in its primary SOC Respiratory, thoracic and mediastinal disorders, and it can also be found with related cardiac symptoms in its secondary SOC Cardiac disorders.
• Include grouping terms (HLGTs, HLTs) when possible (remembering the caveats described in Section 2.5.1).
• In general, queries should be built on PTs and grouping terms. Unless very specific concepts (e.g., bacterial species) are needed, avoid using LLTs to build queries.
• Consider saving the customised query for future use; maintenance is necessary for MedDRA version changes.
• A customised query that may be useful to other MedDRA users can be submitted to the MSSO as a Change request for possible development as an SMQ.
The following documents and tools can be found on the MedDRA website (www.meddra.org):
• MedDRA Term Selection: Points to Consider document (also available on the JMO website www.pmrj.jp/jmo/php/indexe.php)
• MedDRA Introductory Guide
• Introductory Guide for Standardised MedDRA Queries (SMQs)
• Pediatric and Gender Adverse Event Term Lists
• MedDRA Change Request Information document *
• MedDRA Web-based Browser *
• MedDRA Desktop Browser *
• MedDRA Version Report (lists all changes in new version) *
• MedDRA Version Analysis Tool (compares any two versions) *
• Production SMQ spreadsheet
• List of system tools that support SMQs
* Requires user ID and password to access
The following documents can be found on the ICH website (www.ich.org):
• ICH E2D: Pharmacovigilance Planning
6.2 – Membership of the ICH Points to Consider Working Group
6.2.1 Current members of the ICH Points to Consider Working Group
| Affiliation | Member |
| Commission of the European Communities |
Maria Luisa Casini |
| Sarah Vaughan | |
| European Federation of Pharmaceutical Industries and Associations |
Hilary Vass* |
| Christina Winter† | |
| Health Canada | Polina Ostrovsky |
| Lynn Macdonald | |
| Japanese Maintenance Organization | Yutaka Nagao |
| Kazuyuki Sekiguchi | |
| Mitsuru Takano | |
| Reiji Tezuka | |
| Japan Pharmaceutical Manufacturers Association |
Yo Tanaka |
| Hitomi Takeshita | |
| MedDRA MSSO | Judy Harrison |
| Ministry of Health, Labour and Welfare/Pharmaceuticals and Medical Devices Agency |
Yuhei Fukuta |
| Miki Ohta | |
| Daisuke Sato | |
| Makiko Isozaki | |
| Pharmaceutical Research and Manufacturers of America |
Milbhor D'Silva |
| JoAnn Medbery | |
| US Food and Drug Administration | Sonja Brajovic# |
| Christopher Breder | |
| Ministry of Food and Drug Safety, Korea | YuBin Lee |
| Kyung-Eun Yoon |
* Current Rapporteur
# Regulatory Chair
† Former Rapporteur
6.2.2 Former members of the ICH Points to Consider Working Group
| Affiliation | Member |
| Commission of the European Communities | Dolores Montero |
| Carmen Kreft-Jais | |
| Morell David | |
| European Federation of Pharmaceutical Industries and Associations | Barry Hammond†; Reinhard Fescharek† |
| Health Canada | Alison Bennett, Heather Morrison; Michelle Séguin; Heather Sutcliffe; Bill Wilson |
| Japanese Maintenance Organization | Osamu Handa; Akemi Ishikawa; Yasuo Sakurai; Yuki Tada |
| Japan Pharmaceutical Manufacturers Association | Takayoshi Ichikawa; Akemi Ishikawa; Satoru Mori; Yasuo Sakurai; Kunikazu Yokoi |
| MedDRA MSSO | JoAnn Medbery; Patricia Mozzicato |
| Ministry of Health, Labour and Welfare/Pharmaceuticals and Medical Devices Agency |
Tamaki Fushimi; Wakako Horiki; Sonoko Ishihara; Kazuhiro Kemmotsu; Tatsuo Kishi; Chie Kojima; Emiko Kondo; Hideyuki Kondou; Kemji Kuramochi; Tetsuya Kusakabe; Kaori Nomura; Izumi Oba; Shinichi Okamura; Yoshihiko Sano; Nogusa Takahara; Kenichi Tamiya; Daisuke Tanaka; Shinichi Watanabe; Takashi Yasukawa; Go Yamamoto; Manabu Yamamoto; Nobuhiro Yamamoto |
| Pharmaceutical Research and Manufacturers of America | David Goldsmith; Sidney Kahn; Anna-Lisa Kleckner; Susan M. Lorenski; Margaret M. Westland† |
| US Food and Drug Administration | Miles Braun; Andrea Feight; John (Jake) Kelsey†; Brad Leissa; Toni Piazza-Hepp |
† Former Rapporteur
| OTHER TERMINOLOGY PREFERRED TERMS |
No. of EVENTS |
MedDRA Version 17.1 PREFERRED TERMS |
No. of EVENTS |
| Infection | 15 | Upper respiratory tract infection Nasopharyngitis Infection Lower respiratory tract infection Skin infection |
7 2 1 4 1 |
| Abdominal pain | 9 | Abdominal pain Abdominal pain upper Abdominal tenderness |
4 3 2 |
| Accidental injury | 4 | Injury Skin laceration Ligament sprain Back injury |
1 1 1 1 |
Figure 1 – How data coded to a single concept from another terminology may be
expressed by several PTs in MedDRA
| OTHER TERMINOLOGY | MedDRA Version 17.1 | |||
| Reported Event (% subjects) |
Coded Term (% subjects) |
Body System/SOC (% subjects) |
PT (% subjects) |
SOC (% subjects) |
| Hyperglycaemia (4.1) | Hyperglycaemia (10.5) | Metabolism & nutritional disorders (10.5) |
Hyperglycaemia (4.1) |
Metabolism and nutrition disorders (4.1) |
| Increased blood sugar (2.7) | ||||
| Glucose increased(2.2) | Blood glucose increased (6.4) |
Investigations (6.4) |
||
| Blood glucose high (1.0) | ||||
| Increasing glucoses (0.5) | ||||
Figure 2 – Multiple MedDRA terms may be used to code similar medical conditions
included in a “disorder SOC”; associated laboratory findings are in SOC Investigations
| Preferred Terms | Events/Cases | Comment | |
| Version 17.0 | Version 17.1 | ||
| Chest tube insertion | 15 | 0 (no longer a PT) |
In MedDRA Version 17.0, Chest tube insertion was a PT and in Version 17.1 it was demoted to an LLT under PT Thoracostomy. |
| Thoracostomy | 5 | 20 | |
Figure 3 – In MedDRA Version changes - demotion of a PT
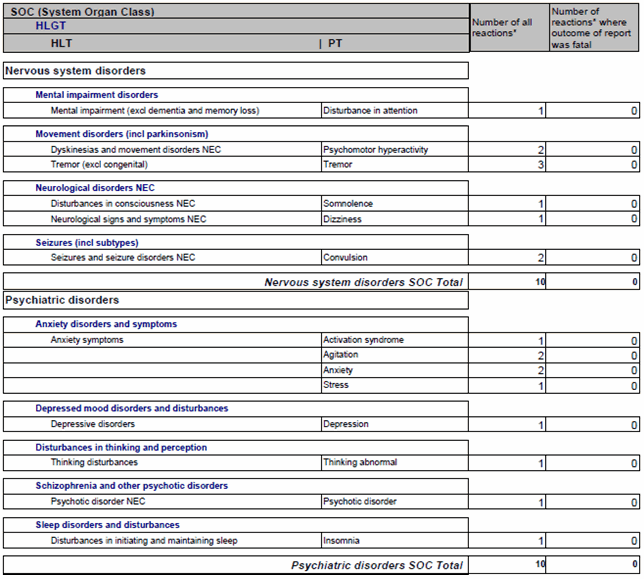
Figure 4 – Primary SOC output listing - example. Note that some PTs are multiaxial, however, this figure shows only the primary SOC assignments
| MedDRA Version 17.1 English Alphabetical Order |
MedDRA Version 17.1 Internationally Agreed Order |
| Blood and lymphatic system disorders | Infections and infestations |
| Cardiac disorders | Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
| Congenital, familial and genetic disorders | Blood and lymphatic system disorders |
| Ear and labyrinth disorders | Immune system disorders |
| Endocrine disorders | Endocrine disorders |
| Eye disorders | Metabolism and nutrition disorders |
| Gastrointestinal disorders | Psychiatric disorders |
| General disorders and administration site conditions |
Nervous system disorders |
| Hepatobiliary disorders | Eye disorders |
| Immune system disorders | Ear and labyrinth disorders |
| Infections and infestations | Cardiac disorders |
| Injury, poisoning and procedural complications | Vascular disorders |
| Investigations | Respiratory, thoracic and mediastinal disorders |
| Metabolism and nutrition disorders | Gastrointestinal disorders |
| Musculoskeletal and connective tissue disorders | Hepatobiliary disorders |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
Skin and subcutaneous tissue disorders |
| Nervous system disorders | Musculoskeletal and connective tissue disorders |
| Pregnancy, puerperium and perinatal conditions | Renal and urinary disorders |
| Psychiatric disorders | Pregnancy, puerperium and perinatal conditions |
| Renal and urinary disorders | Reproductive system and breast disorders |
| Reproductive system and breast disorders | Congenital, familial and genetic disorders |
| Respiratory, thoracic and mediastinal disorders | General disorders and administration site conditions |
| Skin and subcutaneous tissue disorders | Investigations |
| Social circumstances | Injury, poisoning and procedural complications |
| Surgical and medical procedures | Surgical and medical procedures |
| Vascular disorders | Social circumstances |
Figure 5 – The alphabetical SOC order (in English) and the Internationally Agreed
Order of SOCs
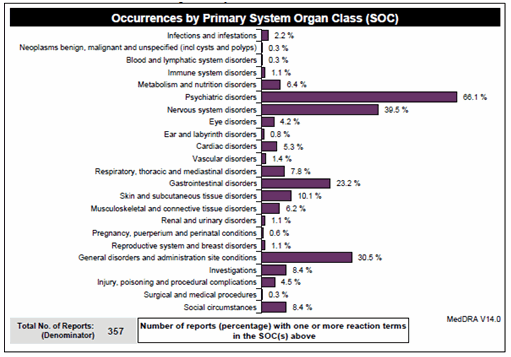
Figure 6 – Example of a graphical display (frequency by primary SOC)
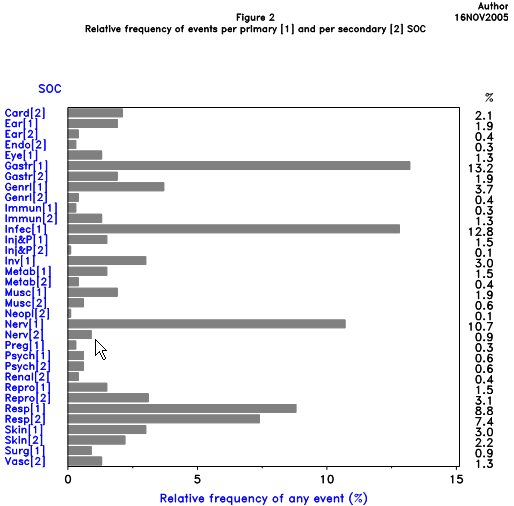
Figure 7 – Example of a graphical display (frequency by primary and secondary SOC)
| System Organ Class | Number of All Reactions* |
Reactions* (% of total) |
Number of Reactions* where outcome of AI report was fatal |
| Gastrointestinal disorders | 1 | 1.92 % | 0 |
| General disorders and administration site conditions | 10 | 19.23 % | 0 |
| Hepatobiliary disorders | 2 | 3.83 % | 0 |
| Immune system disorders | 1 | 1.92 % | 0 |
| Infections and infestations | 1 | 1.92 % | 0 |
| Investigations | 7 | 13.46 % | 0 |
| Metabolism and nutrition disorders | 1 | 1.92 % | 0 |
| Musculoskeletal and connective tissue disorders | 1 | 1.92 % | 0 |
| Nervous system disorders | 10 | 19.23 % | 0 |
| Psychiatric disorders | 10 | 19.23 % | 0 |
| Renal and urinary disorders | 2 | 3.85% | 0 |
| Respiratory, thoracic and mediastinal disorders | 2 | 3.85% | 0 |
| Skin and subcutaneous tissue disorders | 4 | 7.69 % | 0 |
| Total Number of Reactions: | 52 | 100 % | 0 |
Figure 8 - Example of a tabular display (frequency by primary SOC)
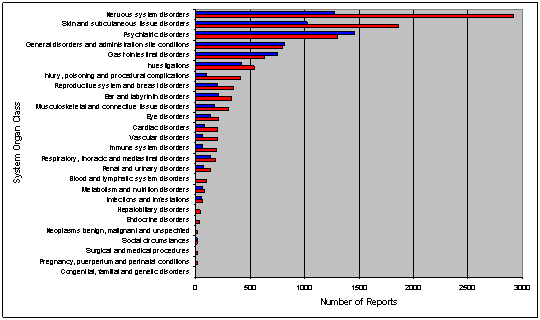
Figure 9a – The upper bar of each pair represents numbers of reports from Consumers (blue) and the lower bar reports from Health Care Professionals (red) (Population 1)
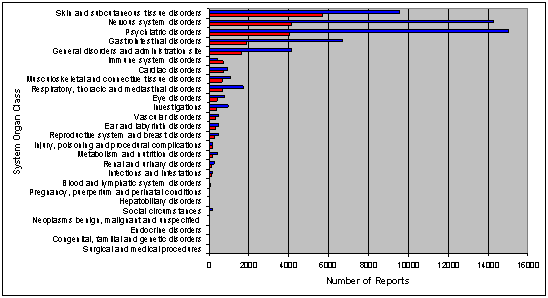
Figure 9b – The upper bar of each pair represents numbers of reports from Consumers (blue) and the lower bar reports from Health Care Professionals (red) (Population 2)
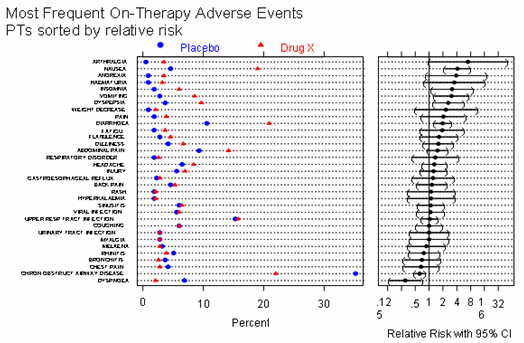
Figure 10 – For a small dataset, a display of PTs may be adequate
SOC Infections and infestations
Primary SOC Analysis
| Adverse Event (MedDRA v17.1) | 25 mg MyDrug (N=44) |
Placebo (N=15) |
| SOC Infections and infestations | 14 (31.8%) | 4 (26.7%) |
PT Upper respiratory tract infection |
5 | 2 |
PT Sinusitis |
3 | 0 |
PT Urinary tract infection |
2 | 1 |
PT Ear infection |
2 | 0 |
PT Viral infection |
2 | 0 |
PT Bronchitis |
1 | 0 |
PT Influenza |
1 | 0 |
PT Localised infection |
0 | 1 |
PT Lower respiratory tract infection |
1 | 0 |
PT Pneumonia |
1 | 0 |
PT Tooth abscess |
1 | 0 |
Secondary SOC Analysis (same data as above)
| Adverse Event (MedDRA v17.1) | 25 mg MyDrug (N=44) |
Placebo (N=15) |
| SOC Respiratory, thoracic and mediastinal disorders | ||
PT Upper respiratory tract infection |
5 | 2 |
PT Sinusitis |
3 | 0 |
PT Bronchitis |
1 | 0 |
PT Influenza |
1 | 0 |
PT Lower respiratory tract infection |
1 | 0 |
PT Pneumonia |
1 | 0 |
| SOC Infections and infestations | ||
PT Viral infection |
2 | 0 |
PT Localised infection |
0 | 1 |
| SOC Renal and urinary disorders | ||
PT Urinary tract infection |
2 | 1 |
| SOC Ear and labyrinth disorders | ||
PT Ear infection |
2 | 0 |
| SOC Gastrointestinal disorders | ||
PT Tooth abscess |
1 | 0 |
Figure 11 – Programmed primary and secondary SOC outputs
Asthma/bronchospasm (SMQ) Cases - Narrow Search (since 1-JAN-2008) |
|||
| ID | MedDRA_PT | REPORT_VERBATIN | DATE_CREATED |
| ----------------------------------------------------------------------------------------------------------------------
|
|||
| 045 | Asthma | Asthma attack | 01-APR-2008 |
| 063 | Asthma | Severe Asthma | 10-JUN-2008 |
| 060 | Asthma exercise induced | Asthma when exercising | 30-MAY-2008 |
| 091 | Bronchospasm | Spasms, bronchial | 12-AUG-2008 |
| 074 | Bronchospasm | Bronchoconstriction | 03-JUL-2008 |
| 100 | Bronchial hyperreactivity | Airways hyperreactive | 20-SEP-2008 |
| 069 | Bronchial hyperreactivity | Reactive airways disease | 21-JUN-2008 |
Asthma/bronchospasm (SMQ) Cases - Broad Search (since 1-JAN-2008) |
|||
| ID | MedDRA_PT | REPORT_VERBATIN | DATE_CREATED |
| ----------------------------------------------------------------------------------------------------------------------
|
|||
| 023 | Allergic respiratory disease | Respiratory (allergy) disorder | 18-FEB-2008 |
| 045 | Asthma | Asthma attack | 01-APR-2008 |
| 063 | Asthma | Severe asthma | 10-JUN-2008 |
| 060 | Asthma exercise induced | Asthma when exercising | 30-MAY-2008 |
| 016 | Bronchial obstruction | Bronchial obstruct | 16-JAN-2008 |
| 039 | Bronchial obstruction | Bronchus obstruction | 14-MAR-2008 |
| 091 | Bronchospasm | Spasms, bronchial | 12-AUG-2008 |
| 074 | Bronchospasm | Bronchoconstriction | 03-JUL-2008 |
| 100 | Bronchial hyperreactivity | Airways hyperreactive | 20-SEP-2008 |
| 069 | Bronchial hyperreactivity | Reactive airways disease | 21-JUN-2008 |
| 088 | Obstructive airways disorder | Obstructive airways disorder | 29-JUL-2008 |
| 049 | Obstructive airways disorder | Obstructed airways dis. | 20-APR-2008 |
| 022 | Wheezing | Wheeze | 16-FEB-2008 |
| 031 | Wheezing | Wheezes | 02-MAR-2008 |
| 106 | Wheezing | Wheezing | 28-SEP-2008 |
| 046 |
Wheezing |
Wheezing (acute) |
06-APR-2008 |
Figure 12 - Results of Narrow and Broad SMQ Searches