

MedDRA MSSO Style Guide1. INTRODUCTION The MedDRA MSSO Style Guide was developed to provide a quick reference guide to aid in the writing process, to ensure a consistent look and feel of the MSSO brand in all documentation.
Correspondence to MedDRA users In addition to these types of documents, CM services are provided for other documents for the purpose of storage and control. These documents, which do not require a full level of CM review and control, should also adhere to the formatting guidelines contained in this style guide. Configuration Management Standard Operating Procedures for the MSSO. MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 21.0. McLean. Virginia. March, 2018 Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Sample References, International Committee of Medical Journal Editors at http://www.nlm.nih.gov/bsd/uniform_requirements.html. Acknowledgements: MedDRA® trademark is registered by IFPMA on behalf of ICH. Microsoft® and PowerPoint® are registered trademarks of Microsoft Corporation in the United States and/or other countries. This section outlines the process for producing an MSSO document. The instructions in this style guide reflect the process for controlled documents; however, the same guidelines can be used for all documentation. Roles and Responsibilities The Author It is the author’s responsibility to: • Request a Configuration Item Identifier (CII) from CM. For revisions, obtain a copy of the document from CM, save as the new document, and change the version number in the filename, title page, and document footer using redline format. • Insert the CII in the footer of the document. Refer to the MSSO document template in the SharePoint Templates_Forms_Logos directory.. • Ensure the Properties screen reflects the appropriate information (i.e., author, organization, title). • Go to File / Options / Trust Center / Trust Center Settings / Privacy Options. The box below should be unchecked • For documents distributed externally, outside the MSSO, remove all personal information from the file after approval of the document.. • Identify the name of the file using the CII (e.g., 000208 Document Name). • Perform an internal review if appropriate prior to finalizing the document • Produce the final document • Ensure all headings, tables, and figures have been entered correctly so that they can be auto generated in the Table of Contents. Do not enter headings manually. Please ensure the Table of Contents is updated throughout the review process to reflect the most current changes. • Ensure the Table of Contents shows MedDRA vs MEDDRA. • Submit the final document to CM for the review and approval process. • The file submitted to CM will be considered the master file. It is recommended that you discard any local copies of the document. If changes are required during the review process, you must use the file that was sent to you by CM during the review, and return the same file to CM. Care should be taken not to change the name of the file prior to returning it to CM • CM will mediate among reviewers and author of the document, to resolve issues brought up during the approval process. • All redlines and comments will remain in the document for the benefit of all reviewers until the document is approved. • If you reject redlines from a reviewer, strike them out and place a comment in the file stating the reason it was rejected. Similarly, if you disagree with a comment, place your name and additional comment within the reviewer's comment field stating the reason for not implementing the comment. • If you agree with a comment, place a note inside the comment block, e.g., "Agree" or "Fixed." Configuration Management • Assign CI Identifiers to the Author during the writing process • Route final documents during the review process until all reviewers approve as published • Maintain configuration status accounting information • Maintain the CM Controlled Document Library. Reviewers • Review the document in a timely manner • Summarize redline changes to the document, summarize the edits in your email to CM, and attach the redlined file • Comments can be used when reviewing a document, but they must be entered using the Word comment feature, or by stating the comment in an email. Do not type your comments into the text of a document. If your comment asks a question, please provide suggested wording changes, if possible. • Return the document to CM using the email that transmitted the document to you. The email contains the review record. • Be sure to keep the review document attached to the email when sending your response to CM. This can be done by forwarding the email to CM rather than replying to CM.. Approval Authority What is in This Section
Usage and Style an hour With the indefinite article, the choice of a or an depends on the sound of the word it precedes. an MSSO Abbreviations With few exceptions, abbreviations should be avoided in formal writing. A few terms, however, are abbreviated in all forms of writing, e.g., Ph.D., B.S., Mr., Mrs., Dr., Prof. Do not abbreviate MedDRA terms. Wrong Surgical SOC Acronyms The first time an acronym appears in the text, expand the acronym and follow it with the acronym in parentheses. Afterwards, use the acronym.
And vs Ampersand (&) The ampersand (&) is regarded as an abbreviation and should be changed to “and” in running text. Exceptions include expressions like “R&D” and names of corporations that are generally abbreviated, such as AT&T, where it would be odd to spell out “and” but not the rest of the abbreviation. Capitalization-MedDRA MedDRA is ALWAYS spelled as shown here. A manual edit may be necessary to retain this format, particularly in Headings and Tables of Contents. The exception would be when you are referencing the original, historic version of “MEDDRA” when the acronym used to stand for “Medical Dictionary for Drug Regulatory Affairs.” Capitalization-Titles In titles (e.g., document titles, PowerPoint slide titles, all headings), capitalize the first letter of all nouns, verbs, and adjectives, as well as prepositions and pronouns that are more than three letters long. Always capitalize the first letter of the first word in a title. Objectives for MedDRA Development
See Section 8.1. Capitalization-Generic Terms Outside of titles, do not capitalize a generic term that follows a proper name: Wrong: He had a Chevrolet Truck. Capitalization-MedDRA Versions When using the MedDRA version in a general sense, do not capitalize; when referring to a specific (named) version, it should be capitalized. Wrong: The upcoming MedDRA Version is scheduled for release in September. (This is not a specific version; therefore "version" should not be capitalized.) Wrong: MedDRA version 8.1 is scheduled for release in September. (This is a specific version and should be capitalized.) Citations When citing sources and references (e.g., journals, books, internet sources, electronic materials) in MSSO published articles, be consistent in the format used. For examples of citation styles, refer to Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Sample References, published by the International Committee of Medical Journal Editors at: http://www.nlm.nih.gov/bsd/uniform_requirements.html. Citations-MedDRA For in-text citing of MedDRA, use "MedDRA Version ##.#." Refer to the Version vs Release Rule section below. For references, use the standard electronic material reference (http://www.nlm.nih.gov/bsd/uniform_requirements.html), which is recommended by Vancouver style: Cancer-Pain.org [homepage on the Internet]. New York: Association of Cancer Online Resources, Inc.; c2000-01 [updated 2002 May 16; cited 2002 Jul 9]. Available from: http://www.cancer-pain.org/. Using the MedDRA Version 20.0 Introductory Guide as an example: MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 20.0. McLean, Virginia [updated 2017 March; cited 2017 Mar 1]. Available from: http://www.meddra.org/how-to-use/support-documentation. Citations-Points to Consider (PtC) documents When citing a section in the Points to Consider (PtC) documents, use one of the following formats depending on the flow: For more information about split coding, please refer to the current release of the MedDRA Term Selection: Points to Consider document, Section 3.5.4. OR Please refer to the current release of the MedDRA Term Selection: Points to Consider document, Section 3.5.4 When to “split” into more than one MedDRA term. In the document TOC itself, the section number is followed by the name without any colons or hyphens. Cities: US cities, Non-US cities When referring to cities, the correct format is as follows: US cities: City, State Country Configuration Item Identifiers The format for the Configuration Item Identifier is: 000208
000208 Configuration Management SOP.docx Data The word “data” is plural for “datum”; the plural form of any verb associated with this word needs to be used. Based on these data and the following assumptions... The data show… Dataset When referring to "dataset," the MSSO has determined that it should be used as one word. Date format Use the international date format for all official and external documentation. The month should not be abbreviated unless space is an issue. Single numeral dates should not have a leading zero. Commas should not be used. Spell out the day of the week if used. Wrong: May 9, 2018 Wrong: 5/9/18 Wrong: 14 Feb 2018 Wrong: 08 August 2018 Wrong: Mon 14, November 2018
Use "1-day" rather than "one day" e.g. vs i.e. The Latin terms e.g. (for example) and i.e. (that is) should be used as shown below.
He enjoys many sports, e.g., swimming, skating, biking.
a SOC must be related directly (i.e., be superordinate) to at least one HLGT.
Bene Factum
Wrong: E.g., "High serum potassium" is considered an investigation.
English spelling All MSSO-generated correspondence and documentation should use North American English spelling. Wrong: The Board greeted the news favourably.
Refer to the Style Guide Annex 3_website guide, located in CM Controlled Documents/Records / Software Tools / MedDRA MSSO Web / MedDRA.org. Font With the exception of certain headers, etc. in MSSO template documents, the default font for all MSSO documents is Arial 12 point. Help Desk "Helpdesk" is not an accepted English word. Always use two words spelled with initial caps. Right: Help Desk Imply and Infer The terms imply and infer are often used incorrectly. The speaker implies while the listener infers. Imply – The instructor implied there would be a lot of written homework. its vs it's “Its” and “it’s” are commonly confused. “Its” is the possessive, referring to something that belongs to “it.” “It’s” is a contraction for the phrase “it is.” An easy way to remember this is as follows: if you are not sure if an apostrophe is needed, say the phrase you wish to write and substitute the phrase “it is.” If the phrase still makes sense, “it’s” can be used. If the phrase does not make sense, use “its.” For example: (It’s) or (Its) a lovely day today!
You can’t judge a book by (it’s) or (its) cover.
Login and Log In Login is the noun (my login) or adjective (the login page), and log in is the verb (you need to log in). Right: My login is entered on the login page MedDRA ID "MedDRA ID" rather than "subscriber ID" or "user ID." MedDRA Terms The correct way to present a MedDRA term is shown below. The term level should precede the term text. Always italicize MedDRA terms, using initial capitals for the first word of the term. This includes naming SMQs in text documentation. Never abbreviate MedDRA terms. For more information, refer to the MedDRA Introductory Guide, Rules and Conventions Adopted in the Terminology. TERM LEVEL Term text
Terms should not be represented in shorthand jargon for any document, email, or formal briefing that goes outside of the MSSO. Wrong: Neoplasm SOC
MedDRA Translations A MedDRA translation should be referred to as “MedDRA language translation” without a hyphen. For example, the Chinese translation should be referred to as “MedDRA Chinese translation” and the German translation should be referred to as “MedDRA German translation.” Multi-axial and Multi-axiality The MSSO preferred style is multi-axial with the hyphen. The MSSO style shall be used except for the MedDRA website, which uses the ICH preferred style, multiaxial, without the hyphen. Numbers Spell out the numbers one to nine unless they indicate a measurement or reflect the appearance of the display: There are five ways to make a list.
Wrong: 5 features are available from this menu.
When a sentence contains 2 or more numbers, and 1 of them is 10 or more, use numerals for each number. The table listed 15 items to be distributed to 3 people.
1.082 Ogic vs ogical In the UK, the suffix “-ogical” is used (e.g., the pathological concept…). In the US, the shorter suffix “-ogic” is used (e.g., the pathologic concept…). Since we use American English in our documents and communications, the shorter suffix should be used. Online The correct spelling is one word, non-hyphenated Right: Online Onsite The correct spelling is "on-site"; however, the MSSO adopts the common, unhyphenated form, "onsite." Right: Onsite training PtC MTS:PTC DRP:PTC When referring to the Points to Consider documents in general, use PtC. The PtC documents are attached.
MTS:PTC MedDRA Term Selection: Points to Consider Screenprints All screenprints should be grammar and spell checked prior to capturing the screen to remove unrecognized words. Care should be taken to remove unwanted underlines. All screenprints and graphics should include a frame around them. To do this: Right click on the image
Shall and will The difference between will and shall is that will makes a promise or a prediction, while shall states a requirement. In general, you should use the word “shall” very carefully as this could be interpreted in some parts of the world as the MSSO being prescriptive and heavy-handed. By tomorrow morning, your work will be done. (a prediction)
Singular vs Plural Staff, Group, Panel. Staff, for all intents and purposes, are singular. There is one staff. Definition 5.e. in Merriam-Webster's entry allows for use as plural, however, the preferred use is singular.
Time format The MSSO has adopted the twenty-four-hour system of expressing time (used in Europe and in the military); four digits always appear as shown below 09:00 = 9:00 a.m.
09:00 USA EDT
User vs. Subscriber The MSSO prefers "User" rather than "Subscriber." Version vs Release Be consistent when referring to our product. The preferred wording is MedDRA Version ##.#. There is a practical reason for doing this. The PtC documents have a “release number” based on the version of the PtC document itself, but they relate to a “version” of MedDRA. Videocast vs Video cast Videocast should be used as one word. Web-Based Browser When referring to the WBB, use a hyphen between "Web" and "Based." All words are initial caps. Wrong: Web Based Browser Website When referring to the internet, use the term web site. When referring to meddra.org, use MedDRA website. Wrong: web site or Web site or MSSO website Which and that Distinguish between the relative pronouns which and that as follows:
The ENTER key, which is located on the left side of your keyboard, is used more than any other key.
That introduces a restrictive clause that is never set off with commas: The ENTER key that is on the numeric keypad cannot be used with this software.
Punctuation The following is a quick reference to basic punctuation rules. Apostrophe Use the apostrophe to make the possessive form: Right: You can view the SOC's title by selecting...
Right: You can view all of the SOCs by selecting... Colon Colons introduce run-in lists and terminate sentences that immediately introduce lists, figures, or tables. Do not end a page with a colon. Comma Commas always appear before the conjunction in lists with more than two items. lions and bears Commas in MedDRA Terms An exception to this rule is the format established for MedDRA terms: SOC Injury, poisoning and procedural complications
Commas used in sentences with two independent clauses The comma always appears before a conjunction that joins two independent clauses that each have their own subject. An easy way to remember this rule is as follows: if removing the “and” would result in two complete sentences, then a comma is needed before the “and.” Wrong: She sat down at the piano and she enchanted the audience. Commas used before Latin abbreviations Use a comma before the Latin abbreviations e.g. and i.e., unless the e.g. and i.e. are used within parentheses. Always use a comma after these abbreviations. Wrong: He enjoys many sports e.g., swimming, skating, biking. Contractions Do not use any form of a contraction in formal English (i.e. documentation meant for official or external use). Wrong: Don’t code to the HLT level. Dash Dashes, also called em dashes, are like parentheses, in that they mark off material that interrupts the syntax of the sentence. Dashes — like parentheses — mark off material… Dashes are weaker than parentheses but stronger than commas. Use dashes to avoid nesting parentheses. Justification With Microsoft Word, all text paragraphs should be left-justified. Hyphens, en dashes, and em dashes Commonly, groupings of two or more words acquire hyphens when used as an adjective or adverb, but remain without when used on their own. Hyphens are also used for hyphenating words for line breaks. En dashes are used between words indicating duration, such as hourly time or months or years; where you might use the word “to.” The long-term aim (adjective) Wrong: October-December Parenthesis Parentheses mark off material that interrupts the syntax of the sentence. Dashes (which are like parentheses) mark off material that…
Nested parentheses should be avoided. Brackets [brackets] should be used. Brackets (which are like [but not the same as] parentheses) mark off material that… Periods Periods end complete sentences. Items in a list are terminated with periods if they are a complete sentence. Place one space after each period. Traditional sentence spacing with two spaces after a period is no longer the accepted standard in most style guides. Quotation Marks In American English, periods and commas always go inside the closing quotation mark; semicolons, colons, asterisks, and dashes always go outside the closing quotation mark; and question marks and exclamation points require that you analyze the sentence and make a decision based on context. “There is nothing to fear,” he said with great emphasis,
“except fear itself.”
”Don’t be absurd!” said Patrick. “To say that ‘I mean what I say’ is the same as ‘I say what I mean’ is to be as confused as Alice at the Mad Hatter’s tea party.”
Semicolon The semicolon replaces an unspoken conjunction. MedDRA Version 8.0 has been published on the web because it is now offered as a downloadable release. Slash Do not form compound words with a slash. If the words are close synonyms, choose an appropriate word and use it instead: Wrong: a preliminary/initial evaluation
Wrong: to view an ADS/TTP… (there is no such entity)
Select ADS/TTP from the Report menu to obtain a report on an ADS or a TTP.
Wrong: an on/off switch
Inclusive or: A or B.
Either A or B.
A and B. Tabs Use tabs and first-line indents. Never use the space bar to align text; never use more than one tab between columns. Adjust the tab settings instead.
Underlining Do not underline for emphasis; use bold or italics instead. Underlining usually refers to a web link within a document or on a web page. Underlining also tends to be heavy, too close to the type, and bumps into the letters. Widows and Orphans Widows and orphans should be avoided. Being aware of a widow – one word on the last line of a paragraph – for us is most applicable to headings and PowerPoint presentations. If possible, bring another word down by using ALT-Shift so that the widow is not left alone. Orphans occur when headings are left on the bottom of a page and the text starts on the next page or when fewer than two lines of text flow over to the next page. Headings should have at least two lines of text after the heading. New pages should start either with a heading or at least two lines of text..
The following templates are available in CM at https://share.mssotools.com/cm/Shared Documents/MSSO Internal NEC/Templates_Forms_Logos/ MedDRA Release Document Meeting Notes for MSSO Personnel MSSO Internal Document MSSO Letterhead MSSO Presentation MSSO Proposal MSSO Training Course Template Broadcast Email Submittal of Broadcast Email
Our standard PowerPoint template is not used for DIA presentations. DIA allows a logo only on the title slide. All subsequent slides must be devoid of logos.
A Copyright is the legal right granted to an author, composer, playwright, publisher, or distributor to exclusive publication, production, sale, or distribution of a literary, musical, dramatic, or artistic work. Copyright symbols should be used only when part of the copyrighted material is used in a document. It is not necessary to use the copyright symbol when referring to a name, without using any of the copyrighted material. A trademark is a distinctive name, phrase, symbol, design, picture, or style used by a business to identify itself and its products to consumers. If the business identified is a service rather than a product, the mark is sometimes called a service mark. A Registered Trademark Symbol® signifies that the trademark or service mark has been officially registered with the relevant trademark registry, with the government. The first time you use the trademark or copyright in a document, you should follow it by the proper symbol. Afterwards, you may use the trademark or copyright without the symbol, as long as you use the correct spelling, capitalization, and punctuation. The trademark™ and registered trademark® symbols should always appear in superscript type, while the copyright © symbol should be in line with the text. You must acknowledge all trademarks and copyrights in a list of acknowledgements in the document.
Drupal® is a registered trademark of Dries Buytaert. Enabler™ is a trademark of Northrop Grumman Corporation. MedDRA® trademark is owned by IFPMA on behalf of ICH. Other product and corporate names may be trademarks, registered trademarks or service marks of their respective owners. 1and1™ and 1&1™ are trademarks of 1&1 Internet, Inc. Acrobat®, Adobe®, Dreamweaver®, Photoshop®, and Reader® are registered trademarks of Adobe Systems Incorporated in the United States and/or other countries. ArisGlobal®, @ris global®, and ARISg™ are either registered trademarks or trademarks of ArisGlobal, LLC. AT&T® is a registered trademark of AT&T Intellectual Property. BSi™ is a trademark of BSi Management Systems America, Inc. Cerner® is a registered trademark of Cerner Corporation. Phase Forward® is a registered trademark of Phase Forward Incorporated. Clintrial™ current owner = Oracle International Corporation. Capability Maturity Models®, CMM®, CMM IntegrationSM , People Capability Maturity Model®, and P-CMM® are registered trademarks of the Software Engineering Institute, Carnegie Mellon University. Dell™and Latitude™are trademarks of Dell Inc. Drupal™ is a trademark of Dries Buytaert Visual InterceptÒ is a registered trademark of Elsinore Technologies, Inc. Enabler™ is a trademark of Northrop Grumman Corporation. Gateway™ is a trademark of Gateway Inc. Gonal-F® is a registered trademark of Merck KGaA. HP®, HP-UX®, Open VMS®, PA-RISC®, Scanjet®, and LaserJet® are registered trademarks of Hewlett-Packard Company. IBM® is a registered trademark of International Business Machines Corporation. Infragistics® is a registered trademark of Infragistics, Inc. InstallShield® is a registered trademark of Flexera Software LLC. Intel® and Pentium® are registered trademarks of Intel Corporation in the U.S. and/or other countries. IONA® and Orbix® are registered trademarks of Micro Focus International PLC. Linux® is a registered trademark of Linus Torvalds in the U.S. and other countries. Mac® and OS X® are trademarks of Apple Inc., registered in the U.S. and other countries. MeSH®, Unified Medical Language System® and UMLS® are registered trademarks of the United States National Library of Medicine. Microsoft Access®, Active Desktop®, ActiveX®, Excel®, FrontPage®, IntelliMouse®, Microsoft®, Microsoft Press®, MS-DOS®, MSDN®, NetMeeting®, Outlook®, PowerPoint®, SourceSafe®, Visio®, Visual C++®, Visual InterDev®, Visual J++®, Visual SourceSafe®, Visual Studio®, Win32®, Windows®, Windows NT®, and SQL Server ® are registered trademarks of Microsoft Corporation in the United States and/or other countries. Novell® and NetWare® are registered trademarks of Novell, Inc. Oracle: Clintrial®, Empirica®, Java®, JavaScript®, Oracle Solaris™, SQL*Plus®, and Oracle® are either registered trademarks or trademarks of Oracle and/or its affiliates. Phase Forward® and InForm® are registered trademarks of Phase Forward Incorporated. Rackspace® is a registered trademark of Rackspace US, Inc. Rebif® (Serono Inc., Geneva, Switzerland). SAS® is a registered trademark of SAS Institute, Inc. SnagIt® is a registered trademark of TechSmith Corporation. Sybase® and SQL Anywhere® are registered trademarks of Sybase, Inc. SNOMED® (Systematized Nomenclature of Medicine) SNOMED CT® (SNOMED Clinical Terms) SNOMED® and SNOMED CTÒ are registered trademarks of the International Health Terminology Standards Development Organisation. System Commander® is a registered trademark of V Communications, Inc. Times New Roman is a registered trademark of The Monotype Corporation. TrueType® is a registered trademark of Apple Computer, Inc. Ultimus® is a registered trademark of Ultimus Incorporated. UNIX® is a registered trademark of X/Open Company, Ltd. Visual Intercept is a registered trademark of Elsinore Technologies, Inc. WebEx® is a registered trademark of Cisco WebEx.
For documents: Disclaimer and Copyright Notice This document is protected by copyright and may, with the exception of the MedDRA and ICH logos, be used, reproduced, incorporated into other works, adapted, modified, translated or distributed under a public license provided that ICH's copyright in the document is acknowledged at all times. In case of any adaption, modification or translation of the document, reasonable steps must be taken to clearly label, demarcate or otherwise identify that changes were made to or based on the original document. Any impression that the adaption, modification or translation of the original document is endorsed or sponsored by the ICH must be avoided. The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document. The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.
Disclaimer and Copyright Notice This presentation is protected by copyright and may, with the exception of the MedDRA and ICH logos, be used, reproduced, incorporated into other works, adapted, modified, translated or distributed under a public license provided that ICH's copyright in the presentation is acknowledged at all times. In case of any adaption, modification or translation of the presentation, reasonable steps must be taken to clearly label, demarcate or otherwise identify that changes were made to or based on the original presentation. Any impression that the adaption, modification or translation of the original presentation is endorsed or sponsored by the ICH must be avoided. The presentation is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original presentation be liable for any claim, damages or other liability arising from the use of the presentation. The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.
Disclaimer and Copyright Notice The information and material provided on this website are protected by copyright and may, with the exception of the MedDRA and ICH logos, be used, reproduced, incorporated into other works, adapted, modified, translated or distributed under a public license provided that ICH's copyright in the information and material is acknowledged at all times. In case of any adaption, modification or translation of the information or material, reasonable steps must be taken to clearly label, demarcate or otherwise identify that changes were made to or based on the original information or material. Any impression that the adaption, modification or translation of the original information or material is endorsed or sponsored by the ICH must be avoided. The information and material are provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original information and material be liable for any claim, damages or other liability arising from the use of the information and material. The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder. __________________________________________________________ Note: We no longer use the Northrop Grumman Logo or copyright on MSSO program documents (e.g., SOPs, Lessons Learned, Program Specifications). The Northrop Grumman logo and trademark statements should be removed from these documents upon updates. The ICH trademark acknowledgement should be used: MedDRA® trademark is registered by IFPMA on behalf of ICH. __________________________________________________________
Japanese Adverse Reaction Terminology (J-ART) is a product of the Ministry of Health, Labour and Welfare (MHLW).
Appendix C. Special Characters in Word 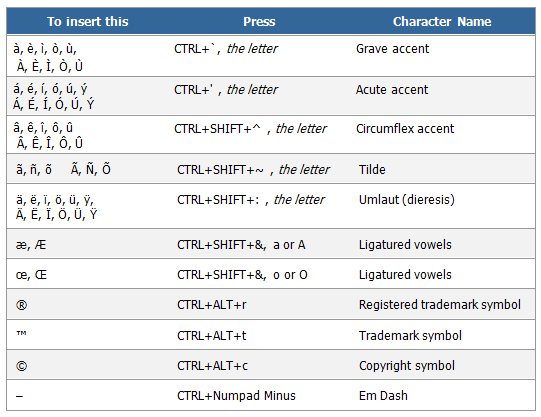 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||